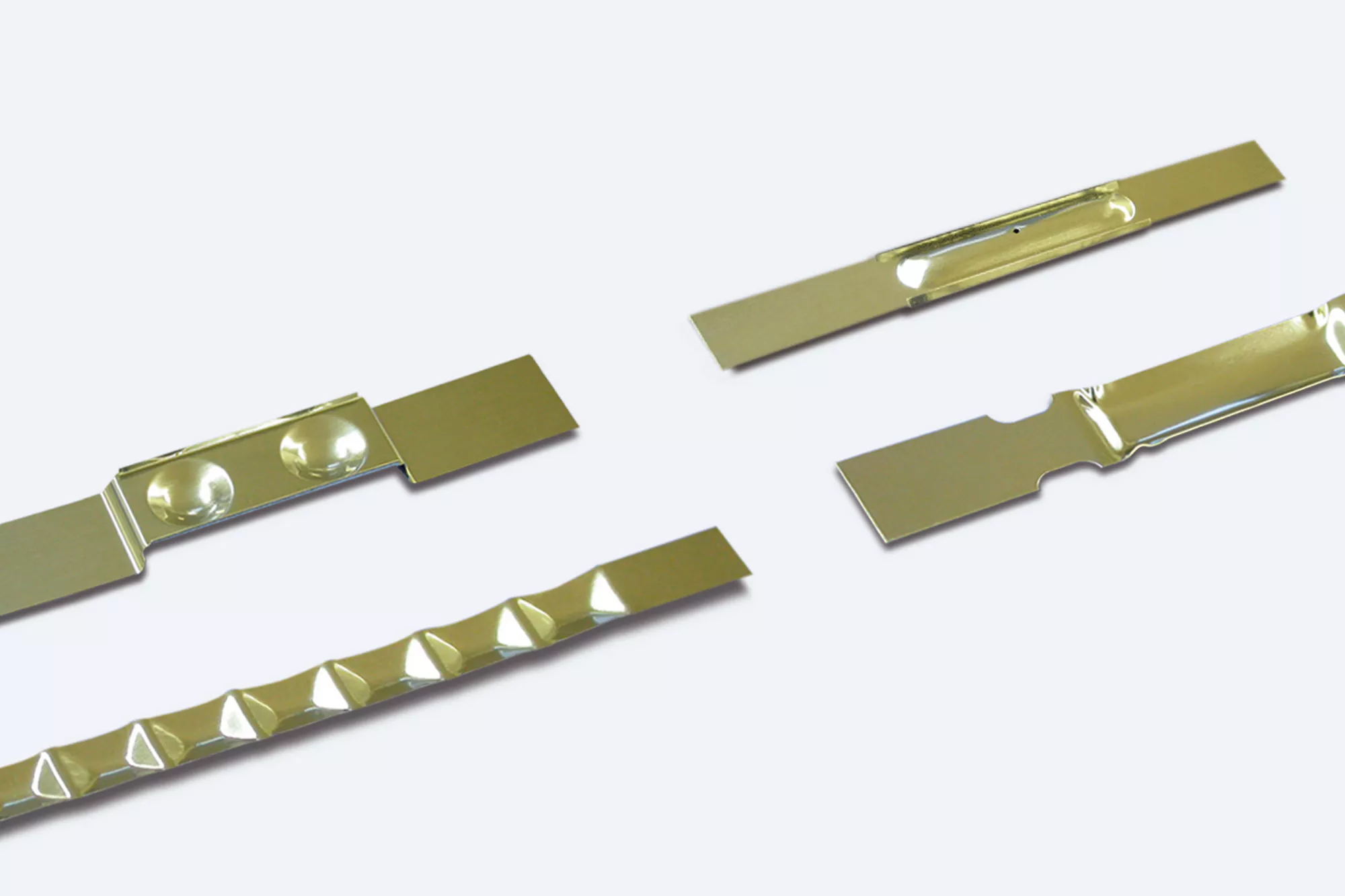
Tantalum is a sensible choice whenever high corrosion resistance is required. Even though tantalum is not one of the noble metals, it is comparable to them in terms of chemical resistance. In addition, tantalum is very easy to work at well below room temperature despite its body-centered cubic crystal structure. This makes it a valuable metal for a wide range of industrial applications. We use our extremely resistant material to make various products, including components for furnace construction, implants for medical technology, parts for ion implantations, and semifinished products.